Table of Contents
Overview – Pain Gate Mechanism
The pain gate mechanism is a critical spinal and supraspinal system that regulates nociceptive transmission. It underlies how pain is amplified or suppressed — explaining why touch can relieve pain and why expectation alters perception. Central to this model are the substantia gelatinosa, descending inhibitory pathways (e.g. PAG–NRM axis), and neurotransmitters like opioids, noradrenaline, and serotonin. It also helps explain maladaptive pain phenomena such as “wind-up”, neuropathic pain, and phantom limb pain — all essential concepts for final-year medical students and clinical application.
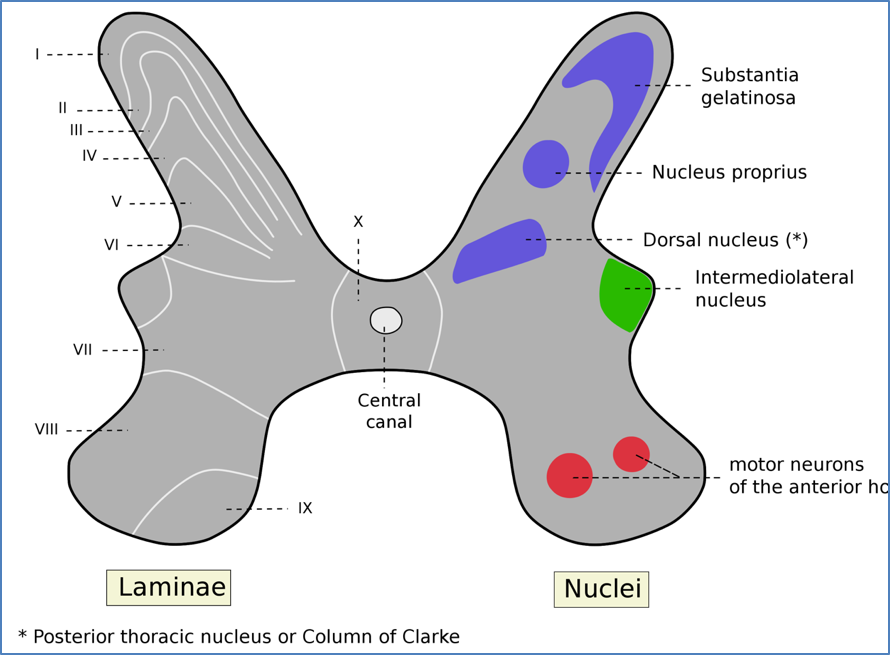
Spinal Modulation – Substantia Gelatinosa
- Gelatinous region at dorsal horn apex
- Key integrator of nociceptive and mechanoreceptive signals
- Gatekeeper for pain transmission
When Activated
→ Inhibits nociceptive transmission
→ ↓ Pain perception
→ Stimulated by:
- Aβ-fibre mechanoreceptor input (e.g. touch, massage)
- Descending inhibitory signals (PAG → NRM → SG)
When Inhibited
→ Allows nociceptive transmission
→ ↑ Pain perception
→ Inhibited by:
- Afferent pain signals (C & Aδ fibres)
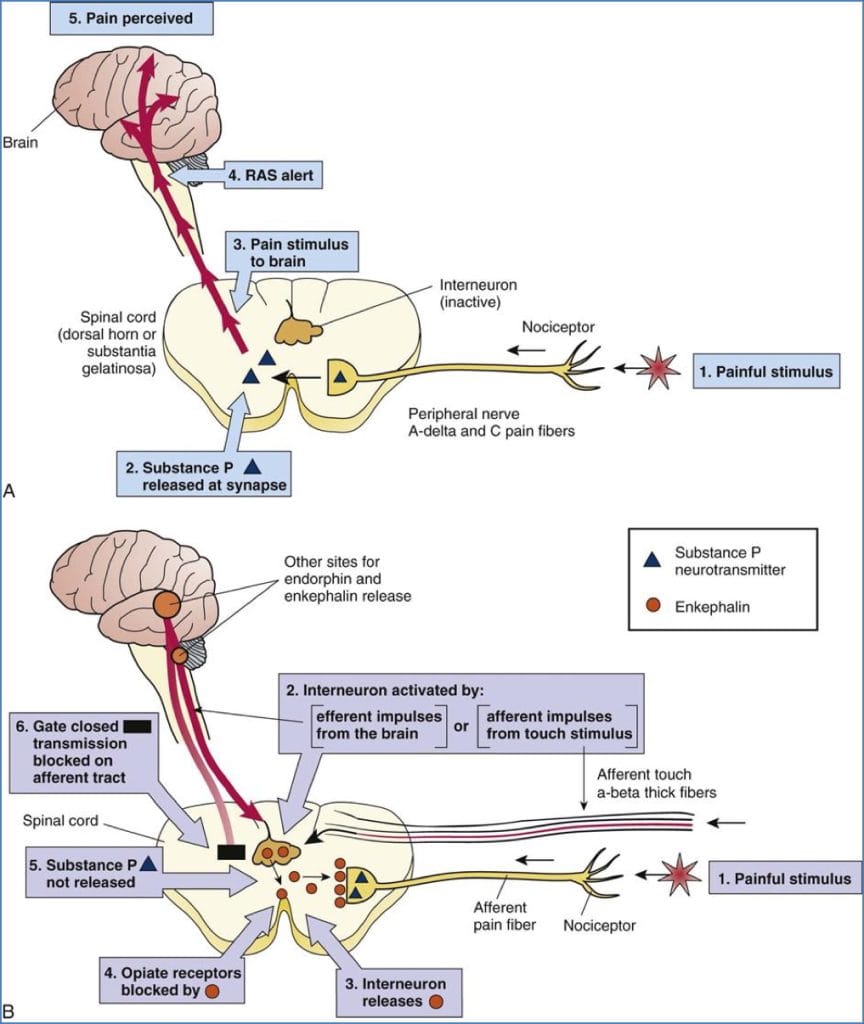
Central Modulation – Descending Inhibitory Pathways
Pathway
Periaqueductal grey (PAG) → Nucleus Raphe Magnus (NRM) → Dorsal Horn/Substantia Gelatinosa
PAG
- Receives inputs from:
- Locus coeruleus
- Reticular formation (RAS)
- Amygdala
- Hypothalamus
- Prefrontal cortex
- Activated by:
- Opioids, GABA antagonists
→ Sends inhibitory signals to NRM
→ NRM → Inhibits dorsal horn synapse & activates SG
NRM
- Acts in two places:
- Direct synaptic inhibition of nociceptive transmission
- Stimulates SG to further suppress pain


Neurotransmitters in Descending Inhibition
- Opioids:
- Remove inhibition of PAG
- Directly inhibit dorsal horn synapse
- Noradrenaline (from Locus Coeruleus)
- Serotonin (5-HT) (from NRM)
- Encephalins
- All directly suppress dorsal horn activity
Tri-cyclic antidepressants block reuptake of NE, 5-HT, & Encephalins → used in neuropathic pain
Wind-Up & Central Sensitisation
Definition
Potentiation of nociceptive synapses between C-fibres and dorsal horn neurons
Process
- Sustained C-fibre input → ↑ release of Substance P & Glutamate
- Synaptic remodelling → ↓ threshold → super-sensitivity
Presynaptic Changes
- ↑ NT release
- ↓ presynaptic inhibition
Postsynaptic Changes
- ↑ excitability (lower threshold)
- ↓ inhibition
Mechanisms
- Phosphorylation of receptors → ↑ function
- ↑ Gene transcription (via 2nd messengers e.g. cAMP) → ↑ receptor proteins
Resolution
- Dephosphorylation by phosphatases
- Protein degradation/replacement restores normal synaptic tone

Brain Processing of Pain
- Pain = central interpretation, not just nociceptive input
- Thalamus → Cortex, Limbic system, Hypothalamus
- Incoming signals are “weighted” vs. other inputs
Influenced by:
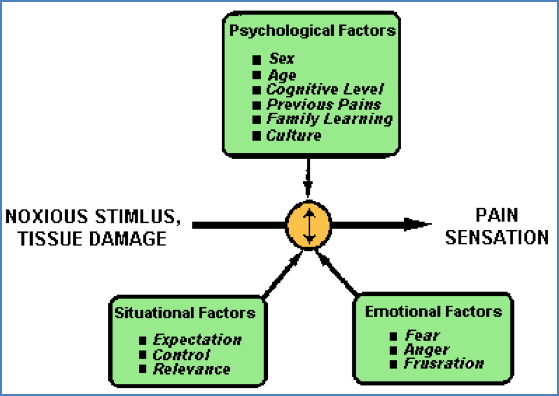
- Attention, emotion, expectation, memory
- Distraction ↓ perceived pain
- Anticipation ↑ perceived pain
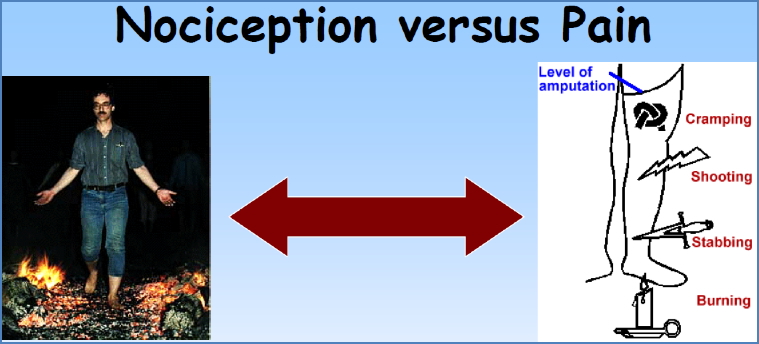
Subjectivity
- Pain ≠ Nociception
- e.g. Firewalkers (nociception without pain)
- e.g. Phantom limb pain (pain without nociception)
Mechanisms of Pain Differentiation
Q: How does the brain distinguish Aδ (fast) vs. C (slow) fibre input?
A: Two hypotheses:
- They synapse on different second-order neurons in the dorsal horn → distinct ascending pathways
- Brain uses signal latency (conduction delay) to differentiate input type
Clinical Relevance – Neural Regeneration
Normal Regeneration
- Mediated by Schwann cells
- Axonal regrowth rate ≈ 1.5 mm/day
- Key steps:
- Axon seals & swells
- Wallerian degeneration (distal axon dies)
- Schwann cells → proliferation, growth factor release
- Regeneration tube forms → guides axon sprouting
- Remyelination follows
- Sprouts culled when target reconnected
Result = functionally restored nerve, but rarely perfect
CNS cannot regenerate

Disrupted Neural Regeneration
1. Neuroma Formation
- Sprout overshoots or misdirected
- → tangled mass of unconnected fibres
- Altered receptor/channel expression:
- ↑ voltage-gated Na⁺ channels
- ↓ activation threshold
- Source of spontaneous discharges → phantom limb pain
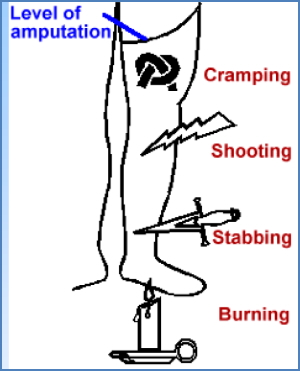
2. Wrong Target Reconnection
- Axon miswires with incorrect ascending neuron
- e.g. Mechanoreceptive Aδ fibre connects with a C-fibre pain pathway
→ Innocuous stimuli now perceived as pain (mechanical allodynia)

Summary – Pain Gate Mechanism
The pain gate mechanism explains how pain perception is dynamically regulated at the spinal and brainstem levels. Key players include the substantia gelatinosa, descending inhibitory pathways (PAG–NRM axis), and modulatory neurotransmitters like opioids and serotonin. Disruption of this system contributes to chronic pain states such as wind-up and neuropathic pain. Neural regeneration is possible in the PNS but may lead to maladaptive wiring and persistent pain.
For a broader context, see our Nervous System Overview page.