Table of Contents
Overview – Haemoglobin and Gas Transport
Haemoglobin and gas transport are central to respiratory physiology. Haemoglobin is the major protein that carries oxygen from the lungs to tissues and also helps transport carbon dioxide back to the lungs for exhalation. Understanding the factors that alter haemoglobin’s affinity for oxygen and the mechanisms of carbon dioxide transport provides essential clinical insight into hypoxia, acidosis, exercise physiology, and respiratory pathology.
Definition – Haemoglobin
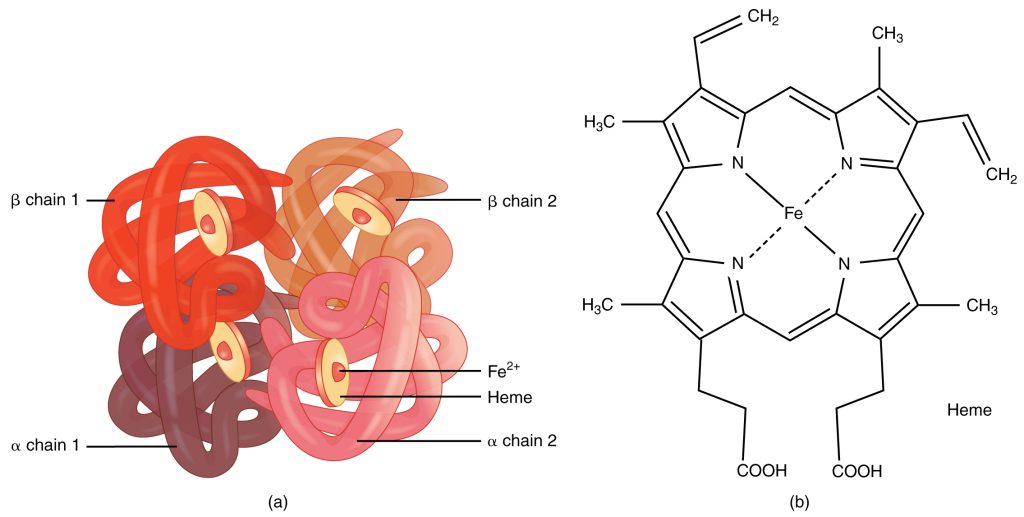
- Haemoglobin is a tetrameric protein molecule composed of four subunits.
- Each subunit contains a heme group with a central iron (Fe²⁺) atom.
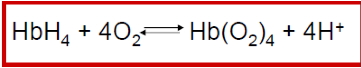
- Each heme can bind one oxygen molecule → one haemoglobin can carry up to four O₂ molecules.
Role in Gas Transport
- Oxygen transport: carries O₂ from lungs → tissues.
- Carbon dioxide transport: also carries CO₂ from tissues → lungs.
- Greatly increases oxygen-carrying capacity of blood by buffering dissolved O₂ and maintaining diffusion gradients.
Oxygen Binding
Cooperative Binding
- Haemoglobin binds O₂ cooperatively:
- Binding of each O₂ increases affinity for the next (up to 4).
- Conformational change between two states:
- T-State (Tense): low O₂ affinity, low saturation.
- R-State (Relaxed): high O₂ affinity, high saturation.
Haemoglobin Saturation
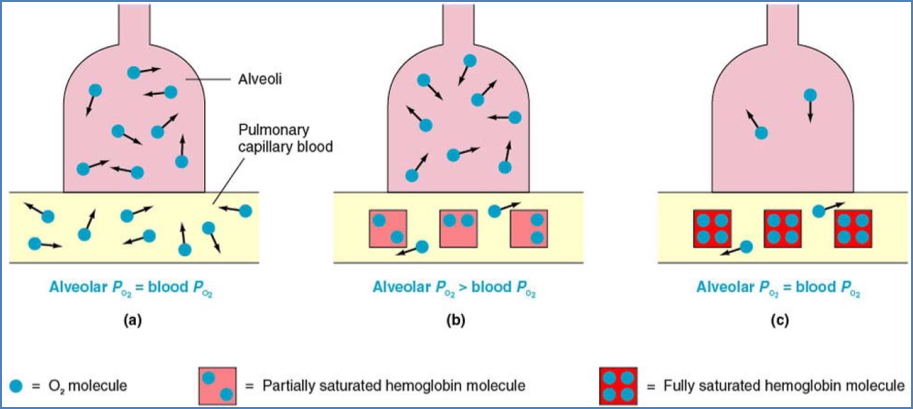
- % saturation depends on partial pressure of oxygen (PO₂).
- Blood O₂ content = dissolved O₂ + Hb-bound O₂.
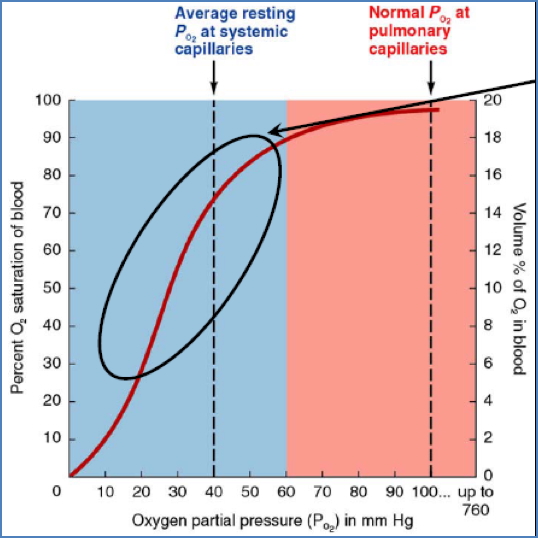
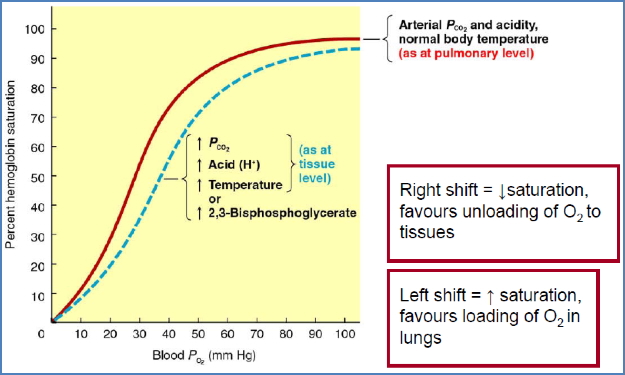
Oxygen-Haemoglobin Dissociation Curve
Plateau Region (O₂ Loading Zone)
- In the lungs (high PO₂, ≈100 mmHg).
- Haemoglobin becomes saturated even if PO₂ falls to ≈60 mmHg.
- Acts as a safety margin for oxygen loading.
Steep Region (O₂ Unloading Zone)
- In systemic capillaries (low PO₂).
- Small ↓ in PO₂ → large ↓ in Hb saturation.
- Favors unloading of O₂ to metabolising tissues.

Shifts in the Curve
- Right shift (favors O₂ unloading, stabilises T-state):
- ↑ Temperature (e.g. exercise).
- ↑ 2,3-BPG (from glycolysis).
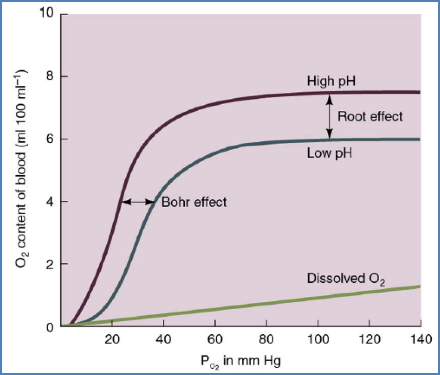
- ↑ PCO₂ (via carbonic acid formation → Bohr effect).
- ↑ H⁺ (acidosis, Root effect).
- Left shift (favors O₂ loading, stabilises R-state):
- Opposite of above.
- Remember: Curve shifts right in exercising muscle.

Factors Altering Haemoglobin Affinity for O₂
- PO₂.
- PCO₂.
- Blood pH.
- Temperature.
- 2,3-Bisphosphoglycerate (2,3-BPG).
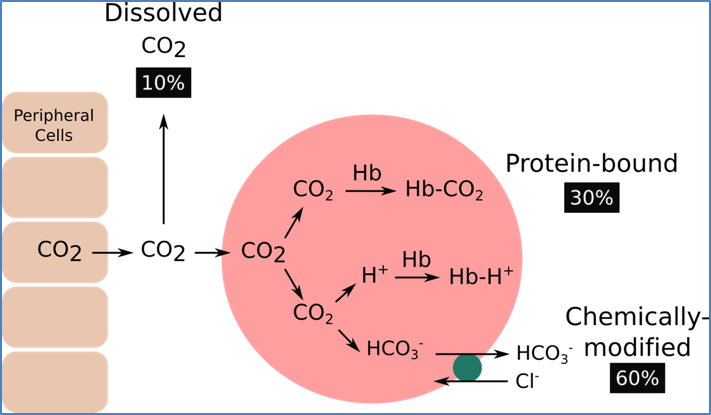
Mechanisms of CO₂ Transport
1. Dissolved in Plasma
- 5–10% of total CO₂.
- Diffuses from tissues → plasma → alveoli.
2. Bound to Haemoglobin
- 25–30% of total CO₂.
- CO₂ binds haemoglobin at a site different from O₂ → carbaminohaemoglobin (HbCO₂).
- CO₂ released in pulmonary capillaries as dissolved CO₂ diffuses into alveoli.
3. As Bicarbonate Ion (HCO₃⁻)
- 60–70% of total CO₂.
- Reaction catalysed by carbonic anhydrase:
- CO₂ + H₂O ↔ H₂CO₃ ↔ H⁺ + HCO₃⁻.
- Bicarbonate exits RBCs → plasma, then re-enters at lungs where it reconverts to CO₂ for exhalation.
Factors Altering CO₂ Transport Efficiency
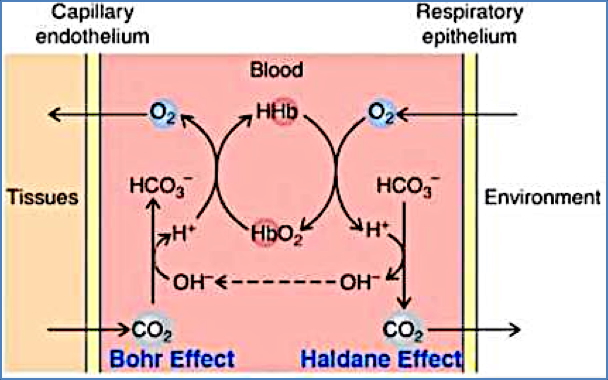
Bohr Effect
- ↑ PCO₂ → ↑ H⁺ (carbonic acid) → ↓ Hb affinity for O₂ → O₂ unloading.
Haldane Effect
- Deoxygenated haemoglobin binds CO₂ more readily than oxygenated haemoglobin.
- Thus, unloading O₂ at tissues facilitates CO₂ uptake, and loading O₂ in lungs facilitates CO₂ release.
Summary – Haemoglobin and Gas Transport
Haemoglobin and gas transport are critical in respiratory physiology. Haemoglobin binds oxygen cooperatively, displays a sigmoidal dissociation curve, and its affinity is influenced by PO₂, pH, temperature, CO₂, and 2,3-BPG. Oxygen loading occurs in the lungs, while unloading occurs in tissues. Carbon dioxide is carried dissolved in plasma, bound to haemoglobin, or most importantly as bicarbonate, with the Bohr and Haldane effects coordinating efficient exchange. For broader context, see our Respiratory Overview page.